Biological
Applications
Liposomes and Lipid Nanoparticles
Liposomes and Lipid Nanoparticles
Liposomes and lipid nanoparticles (LNPs) are very similar in basic physical structure. These are both used as drug delivery vehicles in the body. Traditional liposomes have a lipid bilayer surrounding an aqueous pocket, while LNPs typically only have a single phospholipid outer layer encapsulating the interior, which can be non-aqueous.
As carriers for drug products (DPs), these both provide distinct advantages in that the exterior layer protects the DP from external degradation, and the physiochemical properties of these particles can be optimized for better specificity in the location and rate of drug delivery. For example, techniques such as pegylation can be used with LNPs to boost stability and increase circulation time in order to deliver more efficiently to targeted sites within the body. There is tremendous growth in the use of liposomes and LNPs as delivery vehicles for highly specialized drug payloads, such as mRNA therapies: Both of the initial SARS-CoV-2 vaccines approved for use (BioNTech/Pfizer and Moderna) use LNPs as delivery mechanisms for mRNA DPs.
Accurate Liposome and LNP quantification
One of the distinct advantages of using liposomes or LNPs is that both types of particles can be manufactured with very narrow, uniform particle size distributions. The actual size of the DP delivery envelope can be a critical part of the targeting mechanism for these drugs. Different size particles can target specific organs or tissue types, and, if small enough, can cross the blood-brain barrier to deliver directly to the brain.
This means that the ability to precisely measure particle size distributions for liposomes and LNPs is of critical importance. Spectradyne’s nCS1TM delivers higher-resolution measurement of particle size when compared to optical techniques like dynamic light scattering (DLS) and nanoparticle tracking. At the same time, it also delivers industry-leading concentration measurements, which are also critical because concentration correlates directly to dosage.
Spectradyne’s ARCTM combines the microfluidic resistive pulse sensing of the nCS1 with a particle-by-particle fluorescence detection, allowing phenotyping combined with particle size and concentration measurements. This allows the identification of liposomes through their particle-by=particle fluorescence characterization combined with size and concentration analysis.
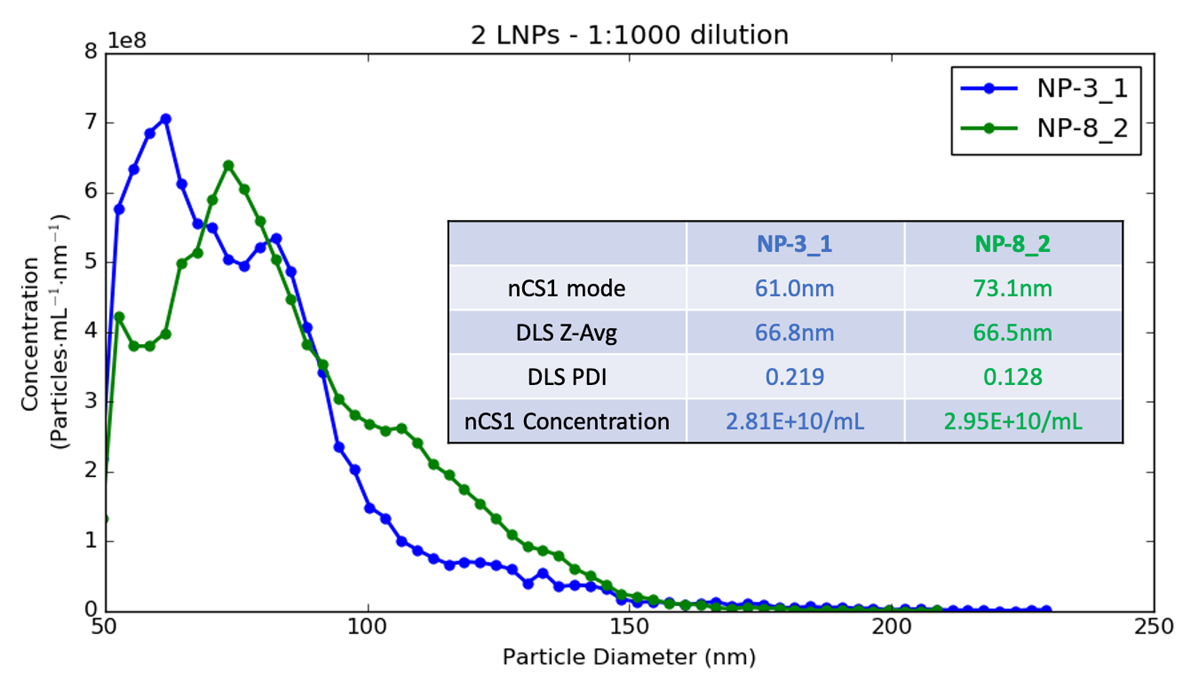
In the plot above, you can see two LNP samples measured using Spectradyne’s nCS1 and also characterized using dynamic light scattering (DLS). DLS was unable to distinguish between the two formulations, which clearly have different mode sizes, and quite different overall distributions. The actual difference detected by Spectradyne’s nCS1 for mode size could affect the ability of the final DP to reach its intended target, so knowing this value exactly is critical for many applications.
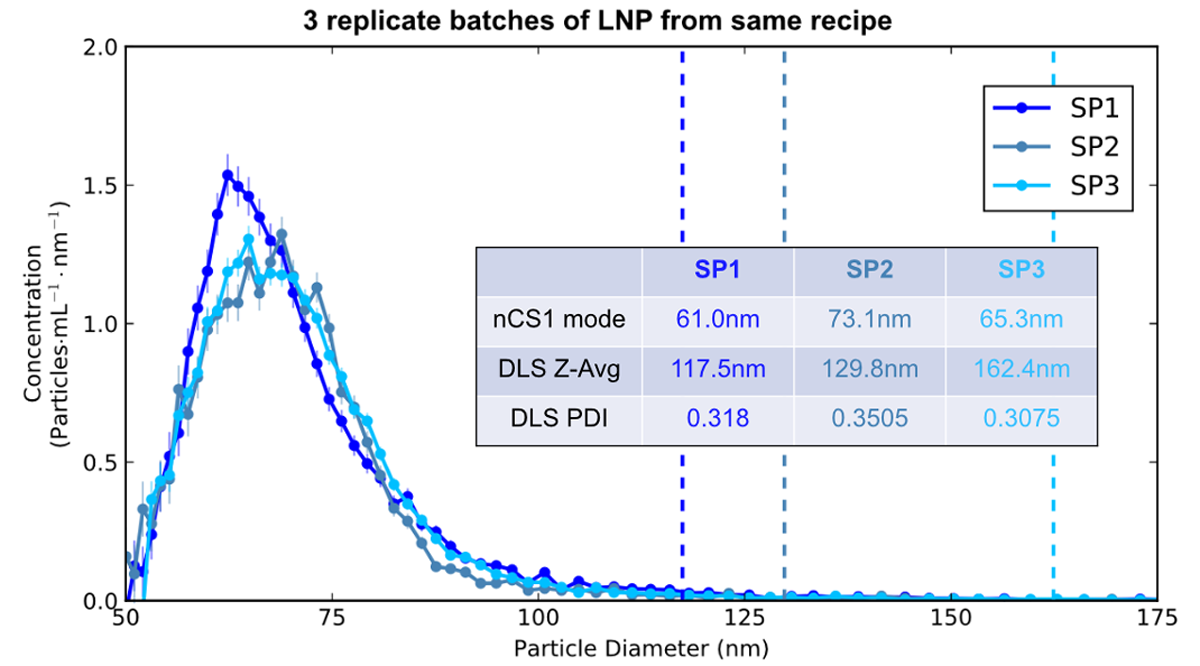
In the second plot above, we are interested in the repeatability of the manufacturing process. The three samples represent three different production cycles for LNPs synthesized using identical recipes and process conditions. The data, taken using Spectradyne’s nCS1, show the three production replicates are very similar in particle size distribution and overall concentration. The DLS data is, by contrast, not usable, as the high bias of light scattering to larger-size particles totally misrepresents the actual sizes, and the high PDIs imply variability that doesn’t actually exist!
The lesson to learn from this: To get the most accurate particle size and concentration methods for your liposomal and LNP-based formulations, you need to use Spectradyne’s nCS1 MRPS system!