Technology
Gas Sorption
Gas adsorption: pore volume and pore size distribution
Gas adsorption for surface and pore analysis offers solutions for pore characterization between 0.3 nm and approx. 500 nm. The determination of BET surface areas and further methods to characterize pores are described on this website as separate methods. In principle the smallest pores are filled first with gas molecules. With increasing pressure successive pore filling of the larger pores takes place. Based on different evaluation models calculations are done to determine pore volumes or pore size distributions. The advantage of gas sorption lies in performing pore analytics of very small pores (micro- and mesopores).
Measuring method
1. Adsorption isotherm
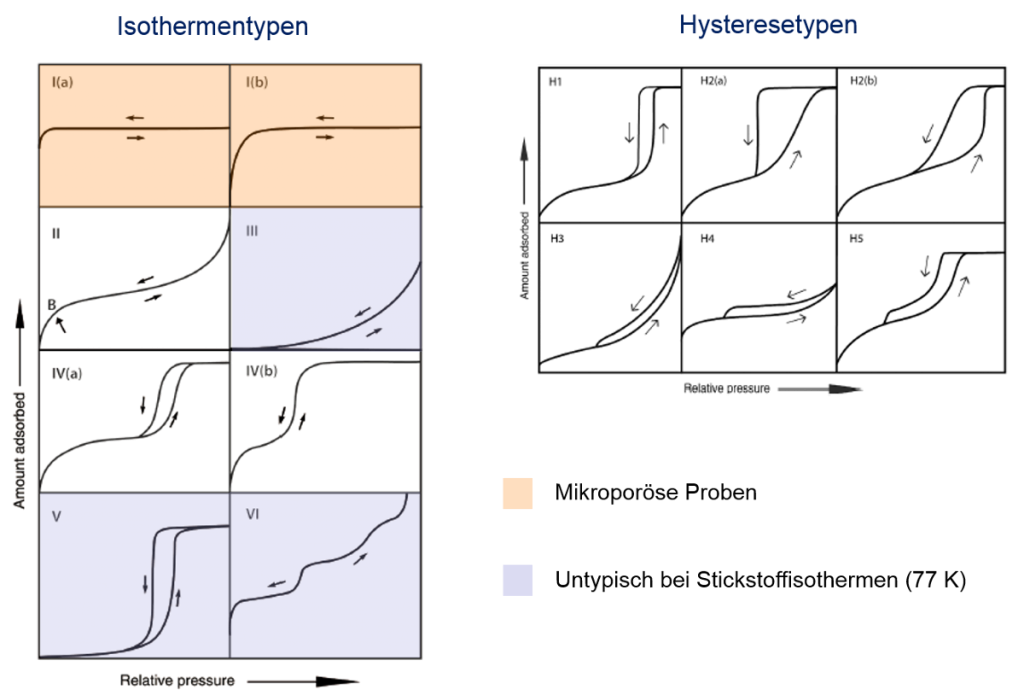
The figure illustrates the classification of isotherm and hysteresis types according to the surface and pore structure of non-porous, micro-, meso- and macroporous materials. Besides BET surface area calculations (see corresponding method on this website) isotherms are used to determine pore volumes (micropore- and total pore volume) as well as pore size distributions. Traditional models are e.g. the BJH method for mesopore analysis, the Gurvich rule for total pore volume calculations or Dubinin equations for micropore analysis. To improve these models different groups around the world develop new calculation models. State-of-the-art models are the so-called DFT (density functional theory) models and Monte-Carlo simulations. In contrast to mercury porosimetry gas adsorption offers different advantages such as mercury-free and easy handling with measuring cells. The measuring range already starts in the micropore range (approx. 0.3 nm) which is not accessible by mercury porosimetry.
Example
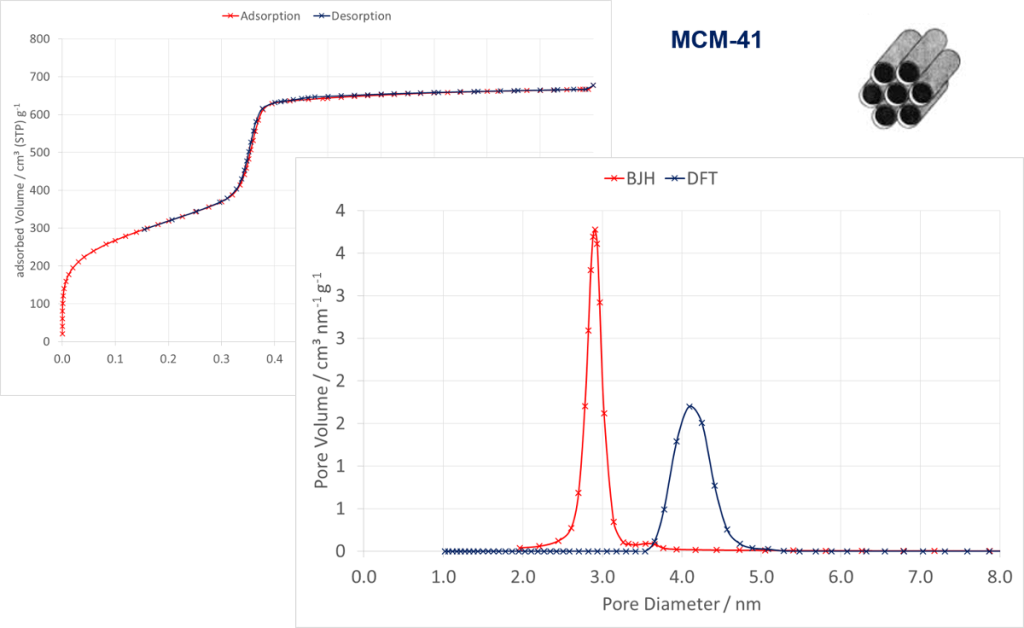
The following figure shows an isotherm of a MCM-41 material measured with Nitrogen at 77 K. The applied evaluation models depends on the type of isotherm and therefore on the kind and ratio of pores. For small mesopores the classical BJH- and modern NLDFT methods were applied. These results show that both methods differ and the real pore size of approx. 4.1 nm could not be calculated exactly by the BJH method. Due to the large number of calculation methods for adsorption isotherms 3P Instruments offers advanced training in terms of surface- and pore characterization analysis to exchange experiences. During this training various topics are discussed such as sample preparation, measuring and interpretation of different kind of solids by means of concrete examples.
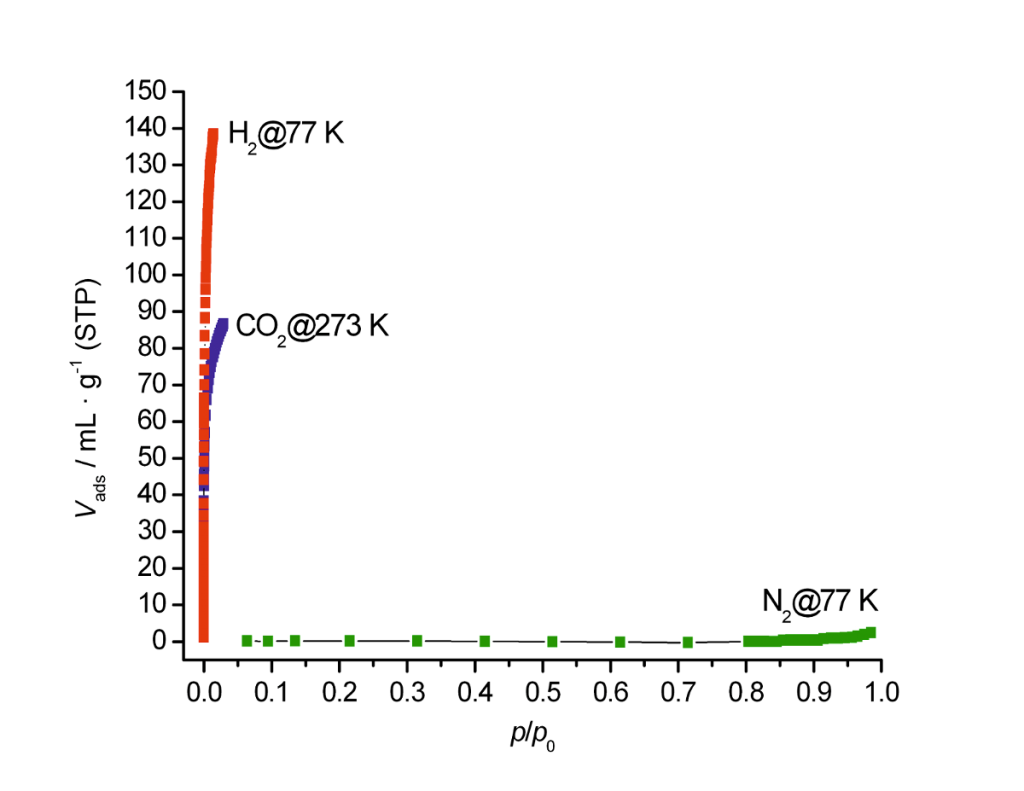
2. Gas adsorption at different temperatures
Pore size analysis by gas sorption is usually done in a relative pressure range between 0 and 1 by measuring the isotherm of gas at its boiling point. Due to the costs and availability of liquid Nitrogen, normally Nitrogen isotherms are measured at 77 K. In principle each gas can be used at different temperatures to investigate the sorption behaviour or to discuss the analysis of the pore structure data also in terms of practical separation processes. Following gas sorption methods have been proved:
- Argon at 87 K for micropore determination according to the IUPAC classifications
- Krypton at 77 K to determine small BET surface areas.
- Krypton at 87 K to analyse small mesopores in thin, porous layers
- CO2 at 273 K to investigate small micropores < 1.5 nm
- H2, CH4, CO2 etc. at different temperatures to investigate gas storage applications various adsorptives at different measuring temperatures to compare adsorption processes or the validation of substance-specific parameters and interpretation models for pore analysis
- Isotherms of an adsorptive at different temperatures to calculate adsorption enthalpies (isosteric heats of adsorption)
- Chemisorption: H2, CO, NH3, pyridine etc. to characterize active surfaces of catalysts
- practical relevant investigation of gas- and vapor mixtures by dynamic sorption methods
Isotherms of Nitrogen at 77 K, CO2 at 273 K and H2 at 77 K were measured on a zeolite 4A. In comparison to hydrogen at 77 K or CO2 at higher temperature, Nitrogen shows almost no adsorption at 77 K. This example shows that Nitrogen measurements at 77 K are not the appropriate method to analyze micropores smaller than 0.5 nm. Other adsorptives than Nitrogen and temperatures have to be used. Our LabSPA (Lab for Scientific Particle Analysis) performs test and contract analyses of different kind of gases at various ranges of temperature and pressure.